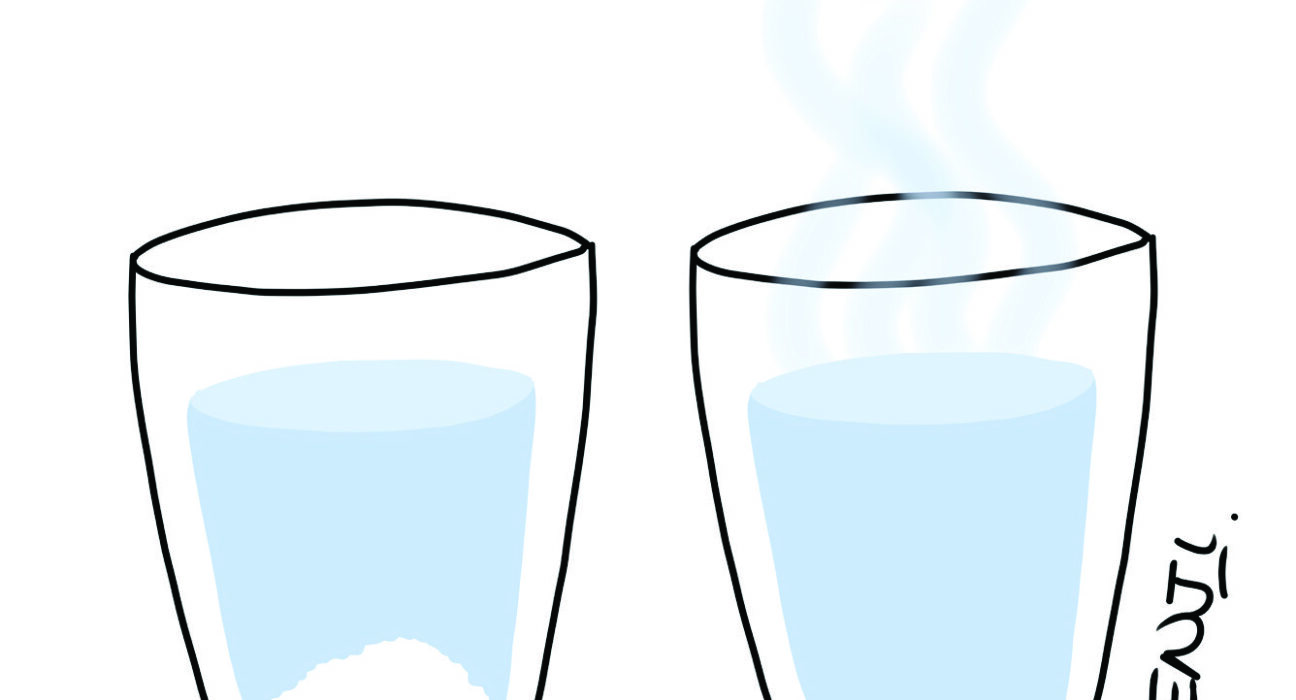
Dissolving Salt
You will a trusted adult to help you boil and handle hot water. This experiment shows that you can only dissolve a certain amount of salt in water and that this amount increases as the water gets hotter
What You Will Need
- Salt
- Cold water in a glass
- Hot water in a glass
- Spoon

Instructions
- Make sure the glasses have an equal amount of water.
- Put a teaspoon of salt into the cold water and stir until the salt disappears. Repeat this process, remembering to count the number of teaspoons you put into the water, until the salt stops dissolving.
- Write down how many teaspoons of salt you could dissolve in the cold water.
- Repeat the same process for the hot water and compare the number of teaspoons of salt dissolved in each liquid.
What Is Happening
The cold water is not able to dissolve as much salt as the hot one. Another name for the liquids inside the cups is a ‘solution’. When this solution can no longer dissolve salt, it becomes a ‘saturated solution’.
The reason the hot water dissolves more salt is because it has faster moving molecules, which are spread further apart than the molecules in the cold water. With bigger gaps between the molecules in the hot water, more salt molecules can fit in between.